Recently, a team of Huang Xingjiu, a researcher at the Institute of Intelligent Machinery of the Hefei Academy of Material Sciences, Chinese Academy of Sciences, and a professor at the University of Science and Technology of China, Zeng Jie, used the Co single atom catalyst (Co SAC) dispersed on nitrogen-doped porous carbon for the first time (III) Ultra-high sensitivity and selective electrochemical detection. At the same time, combined with the methods of synchrotron radiation X-ray absorption fine structure (XAFS) technology, density functional theory calculation (DFT) and kinetic simulation calculation, the interaction between Co SAC and H3AsO3 in the electrochemical analysis process was explored in detail from the perspective of electrocatalysis Function mode and working mechanism. Related results have been accepted for publication by Analytical Chemistry (DOI: 10.1021 / acs.analchem.0c00677).
In recent years, various nanomaterials such as precious metals, alloys, carbon materials, metal oxides and their composites have been widely used in the electrochemical analysis of As (III). However, most studies mainly rely on empirical testing, focusing only on electrochemical phenomena such as sensitivity, detection limit, and selectivity, and ignoring the corresponding detection mechanism. Therefore, we want to truly achieve high sensitivity and selection of As (III) in water Sex testing is still a huge challenge. In fact, the essence of electrochemical detection is the electrocatalytic reaction process at the sensing interface, which will affect the enrichment / reduction of As (III) and the corresponding dissolution / oxidation signal, but there is still a lack of science from the perspective of electrocatalysis understanding. Therefore, to explore the interaction between the sensing interface and the target analyte, and to clarify the corresponding electrocatalytic mechanism, is essential for the development of an effective functional sensing interface to achieve high sensitivity and selective detection of As (III).
Based on the above problems, the researchers first successfully synthesized the Co SAC catalyst and used it for the electrochemical detection of As (III), which can achieve an ultra-high sensitivity of 11.44 μA ppb-1 and excellent selectivity for As (III). This sensitivity is the highest among the currently reported non-precious metal catalysts, and even better than most precious metal nanomaterials. Combining the results of XAFS and DFT calculations, the researchers found that H3AsO3 molecules were catalytically activated by the Co-N2C2 active site during the reaction and formed Co-O hybrid bonds, which led to a decrease in the energy barrier of the As-O dissociation step during the reduction of H3AsO3. In addition, the reaction kinetics simulation calculations show that the first electron transfer process is the rate-limiting step of the entire H3AsO3 reduction, which is much faster on Co SAC than on Co nanoparticle materials (Co NPs), thereby promoting Rapid rapid mass deposition greatly enhances the electrochemical response signal of As (III). The selectivity for As (III) is also attributed to the formation of Co-O hybrid bonds because there is no specific interaction site between Co SAC and ordinary divalent heavy metal ions that do not contain oxygen anions.
This work is the first to use a single atom catalyst to construct a sensing interface for the electrochemical analysis of heavy metal ions, which expands the application of single atom catalyst in the field of electroanalysis. Through advanced XAFS characterization, DFT calculation and kinetic numerical simulation, it is possible to effectively link atoms and electronic structures with macroscopic electrochemical phenomena, indicating that the electrocatalytic capability of the sensing material is in the sensitive and selective detection of analytes It plays a vital role. The study of the working mechanism of Co SAC provides atomic-level catalytic insights and important theoretical guidance for the design of functional sensing interfaces in environmental applications. At the same time, it also provides inspiration for catalytic treatment and removal of such pollutants in the water environment.
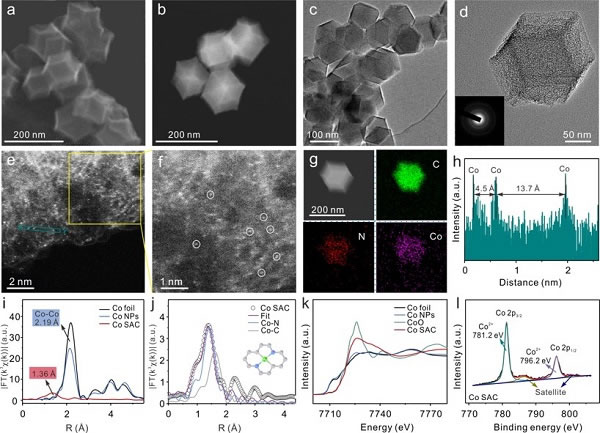
Figure 1. Co SAC material morphology and structure characterization
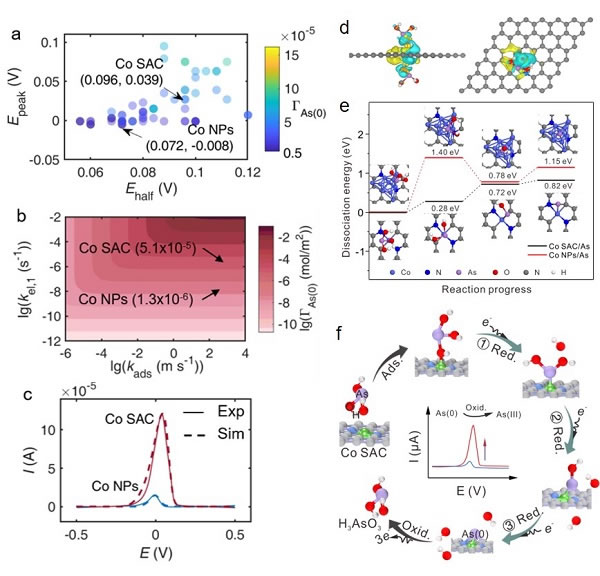
Figure 2. a) -c) Kinetic numerical fitting; d), e) DFT calculation; f) Enhanced electrochemical detection mechanism diagram
Stainless Steel Cutting Board Rack
Cutting Board Rack,Cutting Board steel rack,Cutting Board Organizer rack,Stainless Steel Cutting Board Rack,Chopping Board Rack,Stainless Steel Chopping Board Rack, The materials are used 304 stainless steel, easy to clean, never rust!
Kichen Rack is made of high quality 304 stainless steel, This kind of material steel luxury, never rust, resist corruption, easily clean, safe, healthy and durable. Prevent rust or chemicals from contaminating food and damaging health
Cutting Board Storage Rack,Cutting Board Holder,Stainless Steel Cutting Board Rack,Chopping Board Rack
Shenzhen Lanejoy Technology Co.,LTD , https://www.grill-mesh.com
![<?echo $_SERVER['SERVER_NAME'];?>](/template/twentyseventeen/skin/images/header.jpg)